
Adolescent Depression Screening Instruments: A Review Of Existing Instruments To Screen For Adolescent Depression
Prevention and early detection of adolescent depression is a national health priority and a key recommendation of the U.S. Preventive Services Task Force (USPSTF). Despite current guidelines that recommend conducting routine screening for depression during teen years, policymakers, school administrators, caregivers and other stakeholders express concerns about the implementation and sustainability of school-based screening programs. Of particular concern is the identification and selection of the appropriate screening instrument that can effectively, efficiently, and safely identify students in need of mental health services. Several screening instruments have been developed and validated for school settings (Bernaras et al., 2019; Forman-Hoffman et al., 2016), making the task of instrument selection feasible.
A review of the most prevalent and recommended screening instruments was conducted to better inform policy and practice concerning the selection of the appropriate adolescent depression screening instrument. This paper details key findings from the review.
Key Psychometric Properties of Depression Screening Instruments
In healthcare settings there are typically two types of tests used to assess an individual’s health: diagnostic and screening. While diagnostic tests provide conclusive evidence of the presence of a condition or illness, a screening test is used to detect the presence or absence of a condition or illness early. The goal of screening is to catch and address potential disorders before they become more dangerous or difficult to treat. Screening tends to be less invasive, expensive, and time consuming than diagnostic tests. However, the ultimate value of screening relies on its ability to accurately predict the condition of interest.
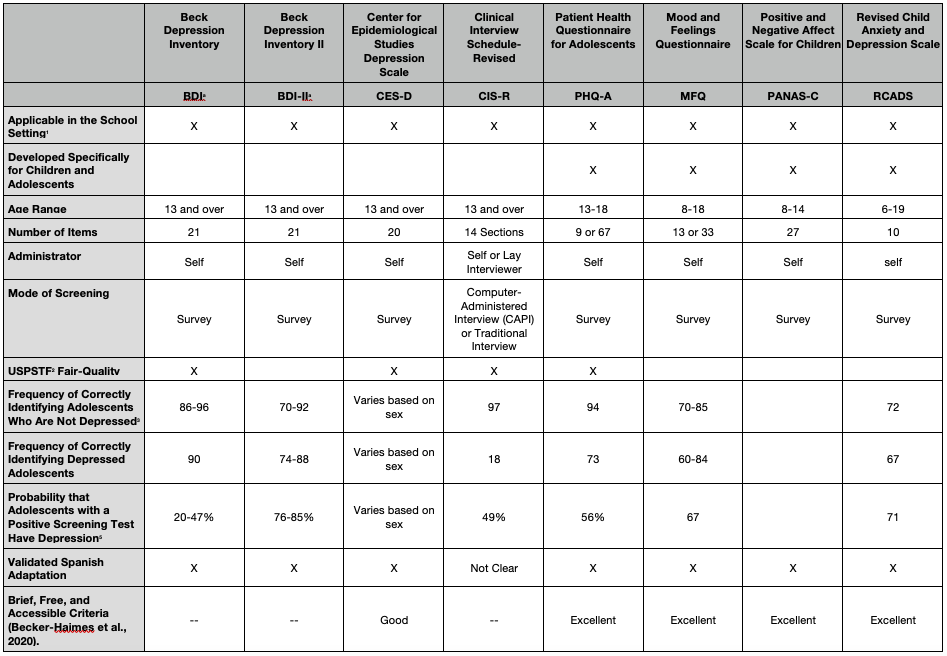
The table below summarizes some of the most widely used, self-administered adolescent depression screening instruments. Those listed include instruments identified by the USPSTF as demonstrating suitable psychometric properties as well as screening tools emanating from research studies. Key psychometric indices included: (a) sensitivity, which is the frequency with which an instrument correctly identifies depressed adolescents; (b) specificity, which is the frequency with which an instrument correctly identifies adolescents who are not depressed; and (c) positive predictive value, which is the probability that adolescents with a positive screening test actually have depression.
Beyond psychometric qualities, it is important that an instrument be accessible, low cost, easily administered, and have its psychometric properties generalize across settings and populations. The table identifies four instruments that met “excellent” criteria for being brief, free, accessible, and validated, according to a recent comprehensive review (Becker-Haimes, 2020). Key indices used in this second review go beyond traditional reliability (consistency) and validity (accuracy) indices and include: (a) prescriptive validity, for which the instrument showed statistically significant accuracy at identifying depression in more than one sample; (b) validity generalization, for which the instrument demonstrated use in multiple demographic groups and in multiple settings; and (d) clinical utility, for which the instrument has demonstrated across multiple studies that assessment data are clinically actionable – that is, data can be used to improve triage, treatment outcomes, and attrition rates.
Evidence-based Screening Instruments
Four instruments met USPSTF’s criteria, including the Beck Depression Inventory (BDI) and BDI II (Cannals et al., 2001; Roberts et al., 1991), the Center for Epidemiological Studies Depression Scale (CES-D; Faulstich et al., 1986), the Clinical Interview Schedule-Revised (CIS-R; Patton et al., 1999), and the Patient Health Questionnaire for Adolescents (PHQ-A; Johnson et al., 2002). Four instruments meeting “excellent” criteria in the Becker-Haimes et al. (2020) review were the Mood and Feelings Questionnaire (MFQ; Angold et al., 1995), PHQ-9 (Johnson et al, 2002), Positive and Negative Affect Scale for Children (PANAS-C; Laurent et al., 1999), and Revised Child Anxiety and Depression Scale (RCADS; Chorpita et al., 2005). Of the USPSTF recommended instruments, Becker-Haimes only reviewed the CES-D, which met “good” criteria.
The evidence supports the availability of free, validated instruments that can be used across settings. Stakeholder (parents, school faculty and administration) fears persist about the potential for breeches in confidentiality, labeling and stigma, and false identification. However, few tangible adverse events have been reported in past school screening efforts (e.g., Fox et al., 2013; Soneson et al., 2018).
2 USPSTF = United States Preventative Services Task Force
3 Specificity:
4 Sensitivity
5 Positive Predictive Value
a BDI is the original screening instrument that identifies symptoms and attitudes associated with depression. The BDI-II is an updated version of the original BDI. It removed four items related to weight loss, body image change, somatic preoccupation and work difficulty and replaced them with agitation, worthlessness, concentration difficulty and loss of energy.
b A second edition of the CDI is also available but was published after included reviews searches. This updated version includes three different protocols for self-report (28-items), teacher rating (12-items), and parent rating (17-items). A validated Spanish adaptation is also available.
